Mandatory compliance regulation from international clients during trade of genetic resources and delay in issuing certifications are major challenges for silk proteins manufacturers.
Bangalore: Hydrolysed silk proteins manufacturers in India are having trouble exporting to countries like the United Kingdom (UK), Germany, Japan and other countries because of delay in certification of compliance to Nagoya protocol adopted during UN Convention on Biological Diversity or COP-10 held in October 2010 in Nagoya, Japan.
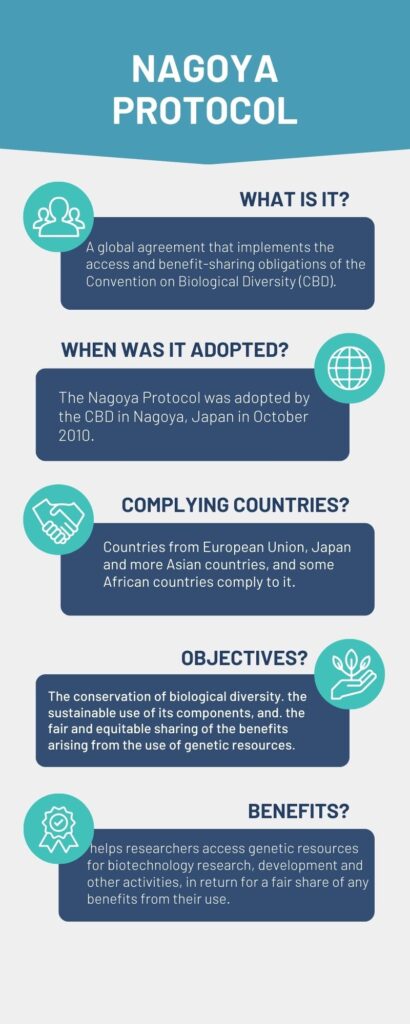
The Nagoya protocol is based on conditions for access to genetic resources and fair benefit-sharing when the genetic resource is exported out of the country. According to Centre of Biological Diversity factsheet on the protocol, one of the major conditions of the protocol is to “provide for issuance of a permit or its equivalent when access is granted”.
Jayant Bhoopalam, Chief Marketing Officer of Serione, one of the manufacturers of silk proteins in India, said that it is a lost opportunity for the businesses in this field. “For every genetic resource produced in the country, even rice or dal, has to comply with the protocol and get a license from the National Biodiversity Authority to export with international clients,” he said.
Bhoopalam added that the two biggest challenges are the tough regulations in other countries and lack of implementation of the Nagoya protocol in India. “In our case, the international client is not accepting the trade because the license in compliance of the protocol is not with us, and silk protein is a small niche when compared to other genetic resources. Since the silk proteins come under both textile and chemical industries, it takes longer to issue the certification,” he said.
Amit Tandon from Seidecosa, a manufacturer of hydrolysed silk protein said that the obligation of providing permit to the manufacturer is not being met, which makes it hard for the export procedure to move smoothly. “We got a license long ago, so export has not been a hassle for us. But newer companies might find it difficult,” he said.
Dr. K.P Raghuram, technical officer (benefit sharing) at the National Biodiversity Authority said that the process of compliance takes at least a month to be certified for international export, be it any genetic resource. “The manufacturer of genetic resource has to sign certain undertakings and fill a form which lists the obligations under Nagoya protocol. After discussions and testing, a license for international exports to the countries complying with the same is issued. This process could take months if issues with forms are found,” he added.
According to a report from Future Market Insights, the silk protein market is expected to grow at about 5.9 percent by 2032, and the market was valued at USD 0.6 billion in 2022.

Dr. Latha R, scientist from Karnataka State Sericulture Research and Development Institute, said that for export of any new plant-based or animal-based product, a certification in compliance with the Nagoya protocol is necessary. She explained that every bio-resource has to get tested and certified to be able to export. “The testing of silk proteins to be used in industries like cosmetics and medicines has been fine and they can be used as an ingredient in products made in India. The issue with international export, must be in the certification process,” she said.




